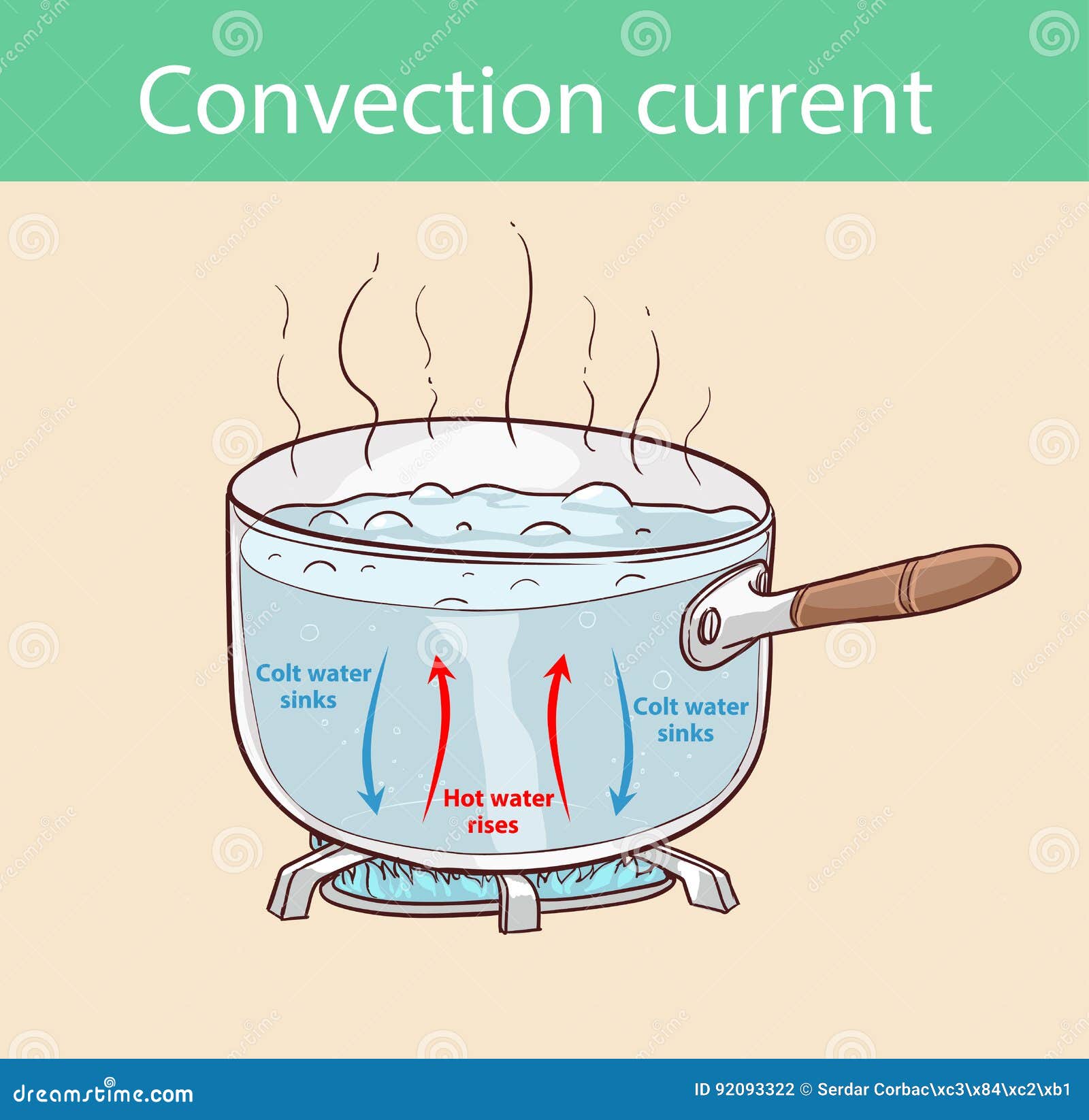
How Much Energy Needed To Boil Water . Phase changes in pure water occur at a specific temperature. i am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c). fresh water freezes at 0° celsius. I didn’t know the exact answer, so i did a little. What difference does it make if you boil more water than you need? this water heating calculator finds the amount of energy and time required to cause a specific water temperature change. can i get the amount exactly right? At 1 atm, water freezes at 0° c and boils at 100° c. the calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h.
from www.dreamstime.com
i am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c). Phase changes in pure water occur at a specific temperature. What difference does it make if you boil more water than you need? the calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). At 1 atm, water freezes at 0° c and boils at 100° c. I didn’t know the exact answer, so i did a little. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h. fresh water freezes at 0° celsius. this water heating calculator finds the amount of energy and time required to cause a specific water temperature change. can i get the amount exactly right?
Diagram Illustrating How Heat is Transferred in a Boiling Pot Stock
How Much Energy Needed To Boil Water this water heating calculator finds the amount of energy and time required to cause a specific water temperature change. this water heating calculator finds the amount of energy and time required to cause a specific water temperature change. At 1 atm, water freezes at 0° c and boils at 100° c. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h. can i get the amount exactly right? I didn’t know the exact answer, so i did a little. fresh water freezes at 0° celsius. Phase changes in pure water occur at a specific temperature. i am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c). the calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). What difference does it make if you boil more water than you need?
From recipes.net
How To Boil Water Without A Heat Source How Much Energy Needed To Boil Water our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h. What difference does it make if you boil more water than you need? Phase changes in pure water occur at a specific temperature. this water heating calculator finds the amount of energy and time required to cause. How Much Energy Needed To Boil Water.
From stovesk.blogspot.com
How To Boil Water Without A Stove STOVESK How Much Energy Needed To Boil Water can i get the amount exactly right? What difference does it make if you boil more water than you need? our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h. fresh water freezes at 0° celsius. the calculators use a simple constant for how much. How Much Energy Needed To Boil Water.
From cebevprd.blob.core.windows.net
How Many Watts Are Needed To Boil Water at David Gordon blog How Much Energy Needed To Boil Water i am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c). our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h. What difference does it make if you boil more water. How Much Energy Needed To Boil Water.
From www.numerade.com
SOLVED A parabolic dish solar cooker is to be used to boil water. It How Much Energy Needed To Boil Water I didn’t know the exact answer, so i did a little. i am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c). our water heating calculator can help you determine both the amount of heat required to raise the temperature of some. How Much Energy Needed To Boil Water.
From www.youtube.com
Thermochemistry Water Phase Change Heat Calculation.wmv YouTube How Much Energy Needed To Boil Water i am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c). this water heating calculator finds the amount of energy and time required to cause a specific water temperature change. can i get the amount exactly right? What difference does it. How Much Energy Needed To Boil Water.
From www.slideserve.com
PPT Heat of Fusion PowerPoint Presentation ID2249917 How Much Energy Needed To Boil Water can i get the amount exactly right? At 1 atm, water freezes at 0° c and boils at 100° c. I didn’t know the exact answer, so i did a little. i am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c).. How Much Energy Needed To Boil Water.
From www.slideshare.net
Energy ch 16 How Much Energy Needed To Boil Water can i get the amount exactly right? At 1 atm, water freezes at 0° c and boils at 100° c. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h. this water heating calculator finds the amount of energy and time required to cause a specific. How Much Energy Needed To Boil Water.
From www.chegg.com
Solved For water, H2O, the heat of vaporization at its How Much Energy Needed To Boil Water What difference does it make if you boil more water than you need? i am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c). fresh water freezes at 0° celsius. Phase changes in pure water occur at a specific temperature. the. How Much Energy Needed To Boil Water.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly How Much Energy Needed To Boil Water What difference does it make if you boil more water than you need? Phase changes in pure water occur at a specific temperature. I didn’t know the exact answer, so i did a little. the calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). i am trying to. How Much Energy Needed To Boil Water.
From www.yiannislucacos.gr
How to boil Boiling as a basic cooking method Yiannis Lucacos How Much Energy Needed To Boil Water fresh water freezes at 0° celsius. can i get the amount exactly right? What difference does it make if you boil more water than you need? our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h. Phase changes in pure water occur at a specific temperature.. How Much Energy Needed To Boil Water.
From haipernews.com
How To Calculate Heat Capacity Of Water Haiper How Much Energy Needed To Boil Water At 1 atm, water freezes at 0° c and boils at 100° c. this water heating calculator finds the amount of energy and time required to cause a specific water temperature change. i am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees. How Much Energy Needed To Boil Water.
From cebevprd.blob.core.windows.net
How Many Watts Are Needed To Boil Water at David Gordon blog How Much Energy Needed To Boil Water can i get the amount exactly right? fresh water freezes at 0° celsius. At 1 atm, water freezes at 0° c and boils at 100° c. this water heating calculator finds the amount of energy and time required to cause a specific water temperature change. I didn’t know the exact answer, so i did a little. Phase. How Much Energy Needed To Boil Water.
From www.numerade.com
The heat of vaporization of a liquid (ΔHvap ) is the energy required to How Much Energy Needed To Boil Water can i get the amount exactly right? our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h. At 1 atm, water freezes at 0° c and boils at 100° c. What difference does it make if you boil more water than you need? the calculators use. How Much Energy Needed To Boil Water.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID5571207 How Much Energy Needed To Boil Water What difference does it make if you boil more water than you need? this water heating calculator finds the amount of energy and time required to cause a specific water temperature change. the calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). fresh water freezes at 0°. How Much Energy Needed To Boil Water.
From www.youtube.com
Energy required reach the boiling temperature of water or to reach 100 How Much Energy Needed To Boil Water I didn’t know the exact answer, so i did a little. can i get the amount exactly right? this water heating calculator finds the amount of energy and time required to cause a specific water temperature change. i am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from. How Much Energy Needed To Boil Water.
From sciencenotes.org
How to Boil Water at Room Temperature How Much Energy Needed To Boil Water i am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees c to 100 degrees c). this water heating calculator finds the amount of energy and time required to cause a specific water temperature change. can i get the amount exactly right? I didn’t know the. How Much Energy Needed To Boil Water.
From cebevprd.blob.core.windows.net
How Many Watts Are Needed To Boil Water at David Gordon blog How Much Energy Needed To Boil Water Phase changes in pure water occur at a specific temperature. the calculators use a simple constant for how much energy is needed to heat water by 1 degree (4168 joules/kg/°c). At 1 atm, water freezes at 0° c and boils at 100° c. fresh water freezes at 0° celsius. our water heating calculator can help you determine. How Much Energy Needed To Boil Water.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? How Much Energy Needed To Boil Water fresh water freezes at 0° celsius. can i get the amount exactly right? our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h. i am trying to calculate the amount of energy required to bring 1 litre of water up to boil (from 20 degrees. How Much Energy Needed To Boil Water.